 Interactions between barley .ALPHA.-amylases, substrates, inhibitors and regulatory proteins
Interactions between barley .ALPHA.-amylases, substrates, inhibitors and regulatory proteins
Authors
SVENSSON BIRTE(Technical Univ. Denmark, Kgs. Lyngby, Dnk) BOZONNET SOPHIE(Technical Univ. Denmark, Kgs. Lyngby, Dnk) BOZONNET SOPHIE(Carlsberg Lab., Copenhagen Valby, Dnk) WILLEMOEES MARTIN(Technical Univ. Denmark, Kgs. Lyngby, Dnk) WILLEMOEES MARTIN(Carlsberg Lab., Copenhagen Valby, Dnk) HACHEM MAHER ABOU(Technical Univ. Denmark, Kgs. Lyngby, Dnk) FUKUDA KENJI(Technical Univ. Denmark, Kgs. Lyngby, Dnk) FUKUDA KENJI(Carlsberg Lab., Copenhagen Valby, Dnk) KRAMHOFT BIRTE(Technical Univ. Denmark, Kgs. Lyngby, Dnk) KRAMHOFT BIRTE(Carlsberg Lab., Copenhagen Valby, Dnk) MAEDA KENJI(Technical Univ. Denmark, Kgs. Lyngby, Dnk) MAEDA KENJI(Carlsberg Lab., Copenhagen Valby, Dnk) HAEGGLUND PER(Technical Univ. Denmark, Kgs. Lyngby, Dnk) FINNIE CHRISTINE(Technical Univ. Denmark, Kgs. Lyngby, Dnk) BONSAGER BIRGIT C.(Technical Univ. Denmark, Kgs. Lyngby, Dnk) BONSAGER BIRGIT C.(Carlsberg Lab., Copenhagen Valby, Dnk) MORI HARUHIDE(Carlsberg Lab., Copenhagen Valby, Dnk) MORI HARUHIDE(Hokkaido Univ., Sapporo) SIGURSKJOLD BENT W.(Univ. Copenhagen, Dnk) ROBERT XAVIER(Ibcp, Lyon, Fra) JENSEN MALENE H.(Ibcp, Lyon, Fra) TRANIER SAMUEL(Ibcp, Lyon, Fra) AGHAJARI NUSHIN(Ibcp, Lyon, Fra) HASER RICHARD(Ibcp, Lyon, Fra)
Journal Title;J Appl Glycosci
Abstract;
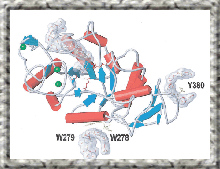
Barley .ALPHA.-amylase binds sugars at two sites on the enzyme surface in addition to the active site. Crystallography and site-directed mutagenesis highlight the importance of aromatic residues at these surface sites as demonstrated by Kd values determined for .BETA.-cyclodextrin by surface plasmon resonance and for starch granules by adsorption analysis. Activity towards amylopectin and amylose follows two different kinetic models, degradation of amylopectin being composed of a fast and a slow component, perhaps reflecting attack on A and B chains, respectively, whereas amylose hydrolysis follows a simple Michaelian kinetics. .BETA.-cyclodextrin binding at surface sites inhibits only the fast reaction in amylopectin degradation. Site-directed mutagenesis and activity analysis, furthermore show that one of the surface binding sites as well as individual subsites in the active site cleft have distinct roles in the multiple attack on amylose. Although the two isozymes AMY1 and AMY2 share ligands for three structural calcium ions, they differ importantly in the effect of calcium on activity and stability, AMY1 having the higher affinity and the lower stability. The role of the individual calcium ions is studied by mutagenesis, crystallography and microcalorimetry. Further improvement of recombinant AMY2 production allows future direct mutational analysis in this isozyme. Specific proteinaceous inhibitors act on .ALPHA.-amylases of different origin. In the complex of barley .ALPHA.-amylase/subtilisin inhibitor (BASI) with AMY2, a fully hydrated calcium ion at the protein interface mediates contact between inhibitor residues and the enzyme catalytic groups in a manner that depends on calcium and which can be suppressed by site-directed mutagenesis of Glu168 in BASI.... (author abst.)
...
Load full article, 163 pages, 480 Kb, PDF file
|

